Researchers in Göttingen have elucidated the structure and function of otoferlin, a protein that plays a crucial role in the hearing process. If otoferlin is missing or its function is impaired, this causes a common form of early childhood deafness. The results, published in the journal Science Advances, mark a milestone after more than two decades of research on otoferlin at Göttingen Campus and contribute to optimizing the first gene therapies for the treatment of deafness.
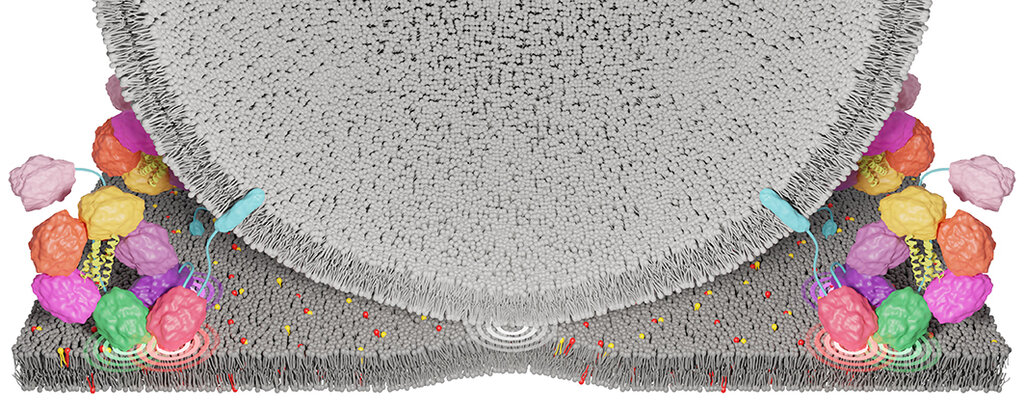
Hearing is a complex process that is still not fully understood today. When a sound hits the hair cells in the inner ear, they begin to vibrate. This movement causes a change in voltage in the cells, which leads to the opening of calcium channels in the membrane. Calcium flows in and triggers the release of a messenger substance. This is transported within the hair cells in small vesicles to the point of contact between the hair cells and the auditory nerve cells, the synapse. Once there, the vesicles attach themselves to the membrane of the hair cells, fuse with it as a result of calcium binding, and release the messenger substance. This activates the opposing auditory nerve cells, which transmit the sound information to the auditory center in the brain.
Key molecule for hearing
The OTOF gene is responsible for the formation of the protein otoferlin, which plays a crucial role in the release of the messenger substance. If otoferlin is missing or its function is impaired, sound information cannot be transmitted to the brain—a condition known as auditory synaptopathy, which is a common form of early childhood deafness. Exactly how otoferlin influences this process has not yet been fully clarified.
Scientists from Göttingen University Medical Center (UMG), the Cluster of Excellence “Multiscale Bioimaging: From Molecular Machines to Networks of Excitable Cells” (MBExC) and the new Collaborative Research Center 1690 “Disease Mechanisms and Functional Restoration of Sensory and Motor Systems” have now succeeded in deciphering the structure and function of otoferlin. The results show that otoferlin has a ring-shaped structure with several binding sites for calcium. The binding of calcium and membrane lipids leads to a change in the otoferlin structure, as a result of which the vesicle “docks” tightly to the membrane, thus preparing for fusion. Otoferlin functions as a calcium sensor for the release of messenger substances: if all binding sites of otoferlin are occupied by calcium, the vesicles fuse with the membrane, a process in which other proteins are presumably involved.
“This is a breakthrough in understanding the molecular basis of hearing. We now have a better understanding of how otoferlin works and why changes in the OTOF gene, known as mutations, lead to protein malfunction,” says Prof. Dr. Tobias Moser, Director of the Institute of Auditory Neuroscience at UMG, spokesperson for MBExC and SFB 1690, and last author of the study. “These new findings are not only fundamental to understanding auditory processing, but also highly relevant to clinical research: The first gene therapy for OTOF mutations has already been successfully tested in clinical trials on the inner ear. Detailed knowledge of the structure and function of otoferlin now opens up the possibility of optimizing these therapies in a targeted manner.”
The results have been published in the journal Science Advances.
Original publication:
Han Chen, Constantin Cretu, Abigail Trebilcock, Natalia Evdokimova, Norbert Babai, Laura Feldmann, Florian Leidner, Fritz Benseler, Sophia Mutschall, Klara Esch, Csaba Zoltan Kibedi Szabo, Vladimir Pena, Constantin Pape, Helmut Grubmüller, Nicola Strenzke, Nils Brose, Carolin Wichmann, Julia Preobraschenski and Tobias Moser. Structure and function of otoferlin, a synaptic protein of sensory hair cells essential for hearing. Science Advances (2025). DOI: 10.1126/sciadv.ady8532
The study in detail
The Göttingen team used a combination of different methods for their investigations.
The structure of otoferlin was examined using cryo-electron microscopy, or cryo-EM for short. To do this, the protein was flash-frozen in a solution and then examined in an electron microscope at minus 196 degrees Celsius. Thousands of individual images of otoferlin were taken under the microscope and then used to calculate a three-dimensional (3D) structure with the aid of high-performance computers. Using these high-resolution snapshots, the researchers were able to visualise otoferlin for the first time in near-atomic resolution – in its free form and in its form bound to artificial membranes. This revealed that otoferlin forms a ring-like structure that is altered by the binding of calcium and lipids and can actively deform the lipid membrane. ‘With the high-resolution structure of otoferlin, we have made the molecular architecture of this unique hearing protein visible for the first time. We were able to see how certain areas of the protein, known as domains, move and interact to enable rapid signal transmission in the inner ear,’ explains co-first author Dr Constantin Cretu, who conducted the structural biology analyses as a junior fellow at MBExC.
Molecular dynamics computer simulations in collaboration with researchers led by Prof. Dr. Helmut Grubmüller, head of the Department of Theoretical and Computational Biophysics at the Max Planck Institute for Multidisciplinary Sciences (MPI-NAT), subsequently confirmed that several C2 domains interact with the membrane simultaneously, enabling synaptic vesicles to dock and fuse.
In cooperation with Prof. Dr. Nils Brose, head of the Department of Molecular Neurobiology at MPI-NAT, it was demonstrated in an animal model that targeted changes in individual calcium binding sites in otoferlin led to faulty sound transmission. In particular, the disrupted calcium binding to otoferlin led to a reduction in the probability of vesicle release when the hair cells were stimulated. A high probability of release is a crucial mechanism for high-precision signal transmission in the ear. “The genetic mouse models have shown us how sensitive the system is to even the smallest changes. Even the failure of individual calcium binding sites led to deficits in the synaptic transmission of sound information – direct evidence of the central role of otoferlin as a calcium sensor for vesicle release,” adds first author Han Chen, who played a key role in advancing the analysis of otoferlin function in the mouse model.
Further information can be found on the websites of the Institute of Auditory Neuroscience, the Cluster of Excellence ‘Multiscale Bioimaging’ (MBExC) and the Collaborative Research Centre 1690.
Contact person for the department:
Prof. Dr. Tobias Moser, Institute for Auditory Neuroscience, Multiscale Bioimaging (MBExC) Cluster of Excellence, Collaborative Research Centre 1690, telephone 0551 / 39-63071, tobias.moser(at)med.uni-goettingen.de, www.auditory-neuroscience.uni-goettingen.de
Press contact:
University Medical Centre Göttingen, Georg August University
Head of Corporate Communications
Lena Bösch
Von-Siebold-Str. 3, 37075 Göttingen
Telephone 0551 / 39-61020
Fax 0551 / 39-61023
presse.medizin(at)med.uni-goettingen.de
You can find the UMG press release (in German) here.